The medicinal use and method of administration dictate how pharmaceutical drugs are manufactured.
The product’s packaging is meant to maintain the drug’s potency and efficacy throughout its shelf life. An expiry date of 90 percent or more significant is the typical rule. The power and effectiveness of a medication can be affected by undesired impurities, and the pace of degradation can be accelerated.
The products are at constant risk of exposure to contaminants during their entire supply chain journey.
- Vapor, moisture, gases, etc., can all create chemical pollution in the air. The product may alter in appearance due to water or vapor exposure. These are all signs that the product is unsuitable for human consumption and should be discarded.
- It is possible, however, for chemical changes to occur without any visible trace. Potency and effectiveness are reduced due to chemical breakdown. This leads certain medicines to generate toxic compounds, which in turn induce unpleasant reactions in patients.
- Bacteria, fungi, and molds are some of the organisms that can cause bio-contamination. Microorganisms are more likely to infect liquid and semi-solid formulations than solid formulations. If unintentionally ingested, microbial growth raises the chance of adverse consequences.
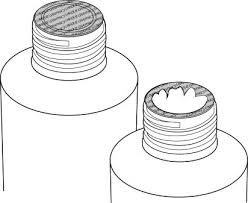 This occurs when the original medicinal product is taken from its container and replaced with an ineffective or fake version. Unaware consumers are exposed to severe risks.
This occurs when the original medicinal product is taken from its container and replaced with an ineffective or fake version. Unaware consumers are exposed to severe risks.
In pharmaceutical packaging, the issue is to ensure that the packaging has no harmful effects on the product and provides a flawless barrier. It is also essential for consumers to know whether the product is safe to ingest since the packaging should be tamper-proof.
Induction Cap Sealing Machine gives a solution by covering the whole mouth of the container with aluminium foil. This barrier effectively keeps contaminants out.
The seal provides even tamper evidence. Supply chains and routes from manufacturers occasionally cross-regional and national borders to consumers. When sealed with an induction cap, the package is durable and can endure transportation and storage.
For more than 18 years, R Technologies has worked with pharma firms to enhance packaging and provide more effective medications to customers.

